Asked and answered over the years for various chemistries

Q. At what rate should I charge my LiIon pack?
A. In general, don't exceed 5C, and best practice is charge at 1C.
Look, teaching you everything you need to know about battery charging
is beyond the scope of this website. We'll share some examples to try
and help guide you, but don't for a minute believe this is a
comprehensive explanation. Point being, it's on you to learn enough to
be safe and the charger manufacturer will also have information to use
in safely charging your battery pack.
To begin, you need to be aware of a bit of math. Like how the 'm' in mAh
means mili, or 1/1000. Regarding the A, that means Amperes (or amps),
and h=hours. So the capacity of the pack is measured amps delivered in
one hour, or Ah (A*h or A x h), but since packs for models are often
measured in fractions of an ampere, then they're also often expressed in
terms of milliamps, or 1/1000th of amp. As for time, since the time
frame is always based one hour, then . . .
- 5000mAh = 5Ah - meaning it'll deliver 5A of current for one hour
- 2000mAh = 2Ah
- 850mAh = 0.85Ah
. . . understand?
Further to this, C (capacity) of a 5Ah pack is 5A for an hour, 2.5A
for two hours. Similarly, 2A of consumption for an hour from a 2Ah pack,
get it?
So charging at 1C (meaning 1xC) it means a 5Ah pack can be charged at
5A, and if the same pack is being charged at 2C (meaning 2xC or 2x5=10)
it means it can be charged at 10A and since we're dealing with full
hours, this means it'll charge in 1/2 that much time, or 1/2 an hour.
Confused? Continue reading.
Let's switch to a 2Ah pack (same-same as a 2000mAh pack). Charging a
fully discharged 2Ah pack at 1C means hitting it with the charger at 2A
for one hour. Hit it at 2C (4A) means it takes half that much time
(1h/2=1/2h or 30-minutes) Ditto, charging at 3C takes 1/3 of an hour or
20 minutes (60min/3=20minutes), or at 4C takes 1/4th of an hour, or 15
minutes. Get it? If not, review and consider learning about this
material in other places until it clicks because if you screw up you're
putting your life at risk.
We're not kidding so please consider yourself warned!
Note; all this is in theory because it's never a good idea to
discharge below 20% capacity. Point being, a 5000mAh pack in practice
should be considered 80% of 5ooomA (0.8 x 5000=4000), or 4000mAh instead
of 5000mAh. This is important!
There's more to learn, and it's not really rocket science, but it's
on you to go learn about it before charging batteries. We're sharing
some some rules of thumb that will for the most part keep you out of
trouble - but - you can burn your house down by being stupid so don't go
trying to blame us because a) we're telling you battery charging can be
dangerous, and b) that what we're sharing isn't everything you need to
know. The major point being, you should go learn how to do it safely
before you begin!

Q. My charger has a LiPo charge-cycle instead of LiIon. May I still use it?
A. Yes.
In general, chargers expressly made to charge LiIon packs are set to
similar cell-voltages for LiPo-chemistry and thus, won't damage the
pack. Basically, the difference internally is the LiIon cell has a
liquid electrolyte while the LiPo, which is also a lithium-ion
technology, uses a gel for the electrolyte. Anyway, always use a charger
designed for the appropriate chemistry. Note; chargers are available to
charge multiple chemistries.

Q. My charger has a LiFe charge-cycle instead LiIon. May I still use it?
A. In general, no for LiIon because a LiFe-charge cycle is going to charge at a lower level than for LiIon.

Q. My battery is marked LiFePO4 instead of LiIon and marked
6.6V instead of 7.4V. My charger has a LiFe charge cycle, may I use
this?
A. Yes. Similar to how you can charge a LiIon with a LiPo
charge cycle, LiFePO4 can be charged with a LiFe charge cycle. And like
LiIon is similar to LiPo in chemistry and different in physical
construction, LiFEPO4 is similar to LiFe with similar chemistry and
different physical packaging (meaning LiFe are packaged in polymer bags
like LiPo, and LiFePO4 within metal cylindrical cells like LiIon.
And
for our purposes (handling and use within models), for pretty much the
same reason; meaning they're more robust and thus, more readily
withstand the knocks of life better. Like what? Shifting within the
fuselage during a crankshaft maneuver, maybe and if it's a soft side
construction, maybe bumping up against he hard edge of a plywood or
carbon fiber former, and getting a ding.
What's important
about this is even if you smooth the ding over with the ball of your
thumb until you can't see it, the ding damaged the electrolyte.
Forevermore, there's a damaged place (maybe now invisible to the eye)
but where heat can build up during charge and discharge.Honestly? This
could mean a fire in your workshop or home. Heads up!

Q.
I've been told LiIon are safer than LiPo and I don't have to worry
about discharging them and storing in fireproof bags. is this true?
A.
There's safer and then there's SAFER. While somewhat less prone to
spontaneously fire than LiPo battery packs, LiIon packs can and do catch
fire. What may lead to this happening? I'm not a battery designer or
chemist, in this instance this is me, John, trying to help guide you
like I was taught by those who knew more than me. So I'm sharing my
experience and trying to help you learn a few of the things I have
learned. Point being, you need to do some research on your own to
confirm pretty much anything and everything you learn, not just here,
but elsewhere. So here's what I know.
Look, chargers can
malfunction and never stop charging. This, maybe leading packs to
overheat and catch fire. We see example of LiIon fires in popular press
stories like this one in the NY Post:
Major
point being, electric scooters and bikes also use these cylindrical
cells (maybe because they fit nicely inside round steel tube frames), so
if I were you, I'd be careful about trusting *any* battery pack. Me? I
store them in fireproof bags of the type you can buy pretty much
anywhere (I buy mine off Amazon). Even then, I store these batteries
inside a .50 caliber ammo can, which I bought at an Army surplus store.
Note; I drilled a 1/4" hole in the lid (because they seal, otherwise).
This, so if one catches fire it can vent the pressure. Next, I epoxied a
bit of stainless steel wool wool on the underside (and over the hole -
but - careful not to plug it with epoxy). The purpose of this is to
serve as a smoke trap and maybe minimize the mess. Does it work? Dunno,
never had a fire. Just relating what *I* do . . . but you do you, as the
saying goes.

Q. Do I need to put my LiFePO4 battery packs at storage mode like I do my LiPo propulsion-packs and my LiIon receiver-packs?
A. No, it's our experience these are the only packs you will
own where it doesn't seem to hurt them to charge today and go fly
tomorrow, or next week, or in six-months! Like if your better-half has
other ideas and the next day while you're loading the truck with models
she announces, 'It's almost spring, take me shopping for painted Mexican terracotta pots, honey!' This, being an example of the now classic, honey-do! So you being a long suffering fellow happily married man, and well experienced in the ways of women, say, 'But of course, Dear, when do you have in mind we should go?"
Saying unlike a LiIon or LiPo which you should soon after deciding
you're not going flying connect to your charger and run a storage mode
cycle, with the LiFePO4 packs I've found you can ignore and use them
when you're good and ready.
Q. I saw a datasheet for a LiFePO4 cell and it refers to them as LiIon, what's going on with that?
A.
Technically, all cylindrical cell batteries are LiIon. It's just that
there are different types so just like there are Lithium-Iron-Phosphate
(LiFePO4 or more commonly in the industry, LFP), there are
Lithium-Manganese-Oxide (LMO), Lithium Nickle Manganese Cobalt Oxide
(NMC), Lithium-Nickle-Cobalt-Aluminum-Oxide (NCA), Lithium-Cobalt-Oxide
(LCO) and Lithium-Titanium-Oxide (LTO), and soon enough, others. And the
one thing they share is a different balance of;
- Cost
- Life Span
- Specific Energy
- Performance
- Specific Power
- Safety
The
best one for Specific Power, Safety, and Life Span is the LFP. And
because these use materials that are comparatively dirt cheap (iron and phosphate instead of nickel and cobalt),
they're less costly. And good and cheap is often a winning combination
in many things, same with batteries in my opinion. The downside is their
Performance and Specific Energy, so LFP cells have less voltage than
other types, 3.2-3.3 versus 3.7-3.8 and this makes them safer and less
prone to spontaneously catching fire.These are good things i my view,
what about yours?
But less voltage isn't as desirable as higher
voltage because servos make more torque and operate faster on higher
voltage (not just ProModeler, all servos, all brands - due to physics,
not marketing) so a caution you should bear in mind is since servos are
marketed by applying lipstick to the pig (again, all servos, not just
ours), they're rated on 8.4V instead of 6.6V because that's where they
shine!
Major point being, if you need 350oz-in and you figure
you're good because you're buying our DS360DLHV servos, think again if
you're planning on running them on a LFP pack because on 6.6V, they
won't make 350oz-in. We're very careful to disclose more than just 6.0V
and 8.4V specs and this is a perfect example of why. The DS360 is rate
at 8.4V and it gives you 360oz-in but at 6.6V it's down to 290oz-in.
Maybe good enough, maybe not. Judgement call.
But . . .
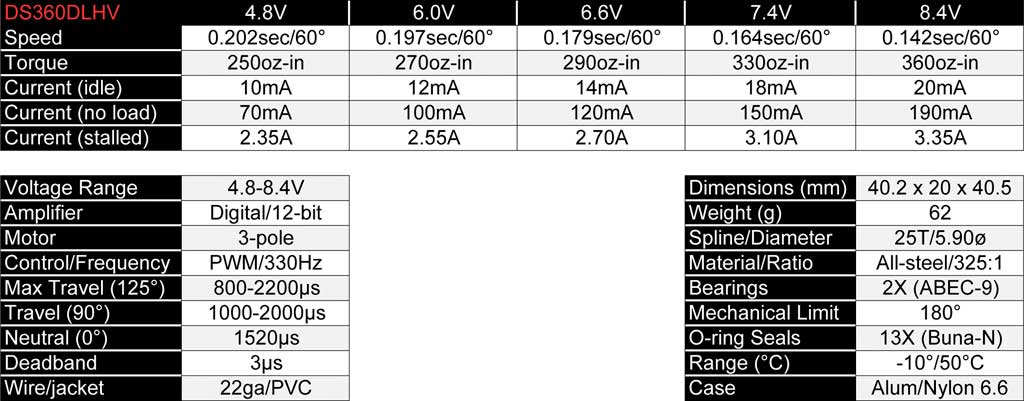
.
. . if you look instead at the specs chart for the DS415BLHV servo
(rated at 8.4V, remember), then at 6.6V it's still making 345oz-in and
that's a *lot* closer to the 350oz-in you want. But, for another ten
bucks, you can, also . . .
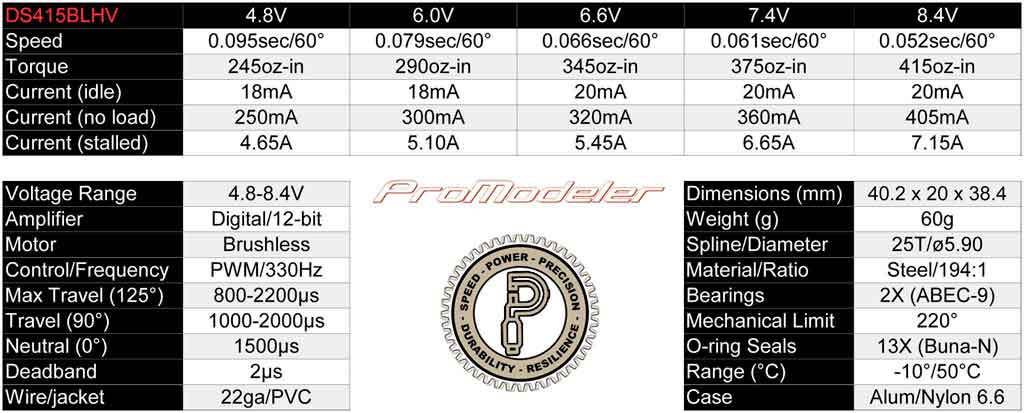
.
. . check out the DS505BLHV servo, which at 6.6V it's still making a
whopping 450oz-in. Me? I like a bit of overkill, what about you?
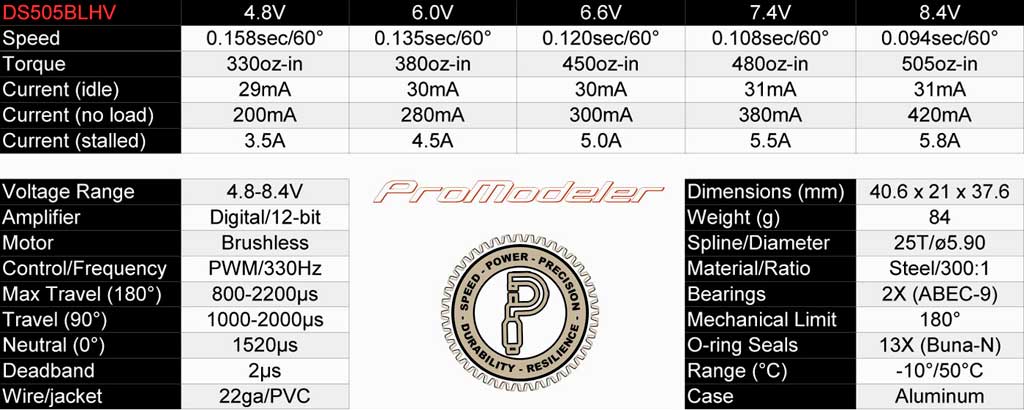
So the take away from this is give consideration to your servos when selecting your battery chemistry. Word to the wise.

Q. Can I charge my 2S LiIon pack with a NiCd charger for 7-cells since that's designed to charge 7.2V packs?
A. Not just no . . . but Hell no! And not just don't do it . . . don't even *think* about it.
The reason is the NiCd charger makes no provision to monitor the cell
voltage of the individual cells the way a charger designed for LiIon
packs will. This is the inherent advantage of the LiIon technology in
that each cell is wired to the little white balance-connector so the
charger can monitor the voltage of each cell as it charges. Be careful
because this is a good way to start a fire and burn down your house!
You've been warned!

Q.
I've heard if I run a LiIon or LFP pack down too low for the charger to
start the cycle, you can unplug the balance connector and set it
NiCd-type in the program and it'll start right up and get some juice
into the pack - enough to start the cycle with the balance connector
connected.
A.
This is true. And if you do it out on the concrete of your driveway
(where if it catches fire there's less risk - but remember, you have to
explain it to the fire department and the insurance company adjuster if
things go teats up), then maybe you can get away with it. Perhaps
5-10-min, where the charger is set to charge a 7-cell NiCd pack (because
7x1.2V/cell equals about the same as an 8.4V 2S LiIon, or as a 7.2V
6-cell NiCd, again where 6x1.2V/cell mimic a 2S LFP), maybe you can get
enough current into the pack to bring the voltage up enough for the
automated charge cycle to take over.
So
yes, I know this may work . .. but riddle me this. What's a model
worth to you, a few hundred bucks, maybe even several thousand? And what
will you say during a deposition when opposing counsel asks, 'Sir,
why would you try to save $30 resuscitating a battery, didn't you
realize it could die unexpectedly, only to lose control and fly through the windshield of you buddy's parked car where his wife was crocheting booties for their granddaughter, and kill her?
Just
saying, there's being smart by saving a few bucks, and then being
really smart and realizing when to cut your loses. Like maybe this is
one of those times when it's smarter to take the battery pack to be
recycled. Just saying, I wouldn't *but* you do you because to your
question, yes, folks have done this successfully.

Q. I'm Canadian and fly year around, sometimes in sub-zero temperatures. Is it OK to charge my LiIon pack in these conditions?
A. Yes, but be careful. Capacity is reduced maybe 20-30% at
freezing. And at lower temperatures the data is inconsistent. Look,
batteries basically like to 'live' at similar temperatures where 'we'
like to live. Fortunately, in practice, what most folks do is charge
their packs whilst in their car!
That said, if you do charge in below
freezing temperatures, reduce the rate of charge to 0.1C . . . e.g. 10% of
the battery capacity.

Q. I'm confused, isn't LiIon the same as LiPo? Also, why don't
you recommend LiPo packs? I like that they're cheap so what's wrong
with that?
A. Yes, LiIon and LiPo are similar. But critical differences aren't so much in their chemistry (they're actually very
similar) but in their methods of construction. This is the key to understanding our recommendation for LiIon versus LiPo.
This is because the LiPo is built in a polymer
bag. This gives it the characteristic brick shape as the
individual cells are flat-rectangles, which are overlaid upon one another. The shape is also the giveaway for
the LiPo vs. LiIon where these packs are built within cylindrical metal shells (typically aluminum).
Note; the Po in LiPo refers to the polymer in it's construction
(aluminized polymer bags). Anyway, the individual cylindrical
shells, because they're made of metal instead of thin polymer bags
means they're more resistant to physical damage. By the way, this metal
shell is the same technology used in old school NiCds and
NiMH (and alkaline cells, for that matter). It's been around forever
because it works!
There are downsides to these metal shells. First, the metal is a bit heavier than the plastic bag use in LiPos. Second, simple
geometry dictates two cylinders contain less volume than two flat cells
(capacity). Third, they're more expensive to produce.
Against these
disadvantages are upsides. Like metal shell is FAR more sturdy. This
turns out to be a crucial advantage because metal protects better
against inadvertent damage (like a pack shifting during a
maneuver and bumping up against the hard edge of a former). If this
happens to polymer style packs, the dent may result in it puffing. Or in
a fire. Need I mention our models are constructed of
flammable materials like balsa, foam, and fiberglass?
Bottom line? For an engineer, part of the remit is looking not at when
everything is
going right, but when things are going wrong. Look, nobody sets out to
install
their pack so it's dented due to shifting during a maneuver, but . . .
shit happens, right? So it's when things go pear shape that a good
engineer earns his pay.
Our deciding against continuing to offer 'Po' style packs for control
avionics is a direct result of data indicating it might sound good in
theory, but in practice, leaves something to be desired. This reminds of the immortal words of a wise wag of baseball.
Put another
way, when the data changes, we change our mind! This is why our control
avionic pack recommendation is to use durable LiIon instead of more
fragile and less costly alternatives like LiPo brick style packs.

Q. How did you come to make batteries with two leads?
A. I've mentioned how and why. before . . . now for the rest of the story.
Way
back in the day, going back on the order of 50 years ago, I flew a Lou
Andrews Aeromaster (53" wingspan). Loved that model flew it for many
years. Re-covered it twice - thank goodness for K2R spray. Powered by my
Lee Custom K&B .61 on SIG 5% with 2% added castor and turning a
12x6, it was a delightful model until it met an untimely demise due to
switch failure. This crash led me to develop a two-lead battery pack.
This
all aided by Mr. Kraft of Kraft Systems who generously agreed to sell
me 25 of their 4-cell NiCd 500mAh battery packs, to include 25 extra
leads (no other source for a Multicon connector since that was their
proprietary product), and he even included a sheet of gold foil labels
with which to reseal the case halves like brand new for after I soldered
on the 2nd lead. I was on my own for finding a larger rubber grommet
but he even lent me a hand with this by including a small bag of servo
mounting grommets. And he gave me some round Kraft patches to hand out,
too.
All this in part because (and I am reading into his actions
what I *think* were the reasons). Meaning, I think he, a) didn't want to
complicate his life with another SKU (stock keeping unit in the
parlance of product sales), and b) believed his switches were reliable
enough, and looking back, c) it maybe tickled him to help start someone
(me) get a start in a business career.
Me? I will remain forever
grateful to his largess to a snot nose kid with a business idea because
he could have blown me off. Unfortunately, when I went off to college,
my mom wanted the room for whatever and tossed pretty everything that
remained of my life there. By this meaning my paperback science fiction
books (I've subsequently replaced my Heinlein, Clark,and Asimov and
added to them the rest in print (except Asimov as I don't have that much
money). She also tossed things like my sandpaper collection stored
within an accordion file my grandfather had given me (embossed with his
law office logo, and of course, irreplaceable), plus my correspondence
with Mr. Kraft, which at the time i didn't attach much importance to. So
basically, pretty much everything I hadn't taken with me to university -
to include my modeling tools - like sanding blocks, X-Acto, Zona saw,
model razor plane, workbench, etc. went into the ash bin as she
basically erased me like I was never there. And looking back, this
sounds bad but she wanted the room and all my stuff looked like just
trash to her broom!
Sounds worse than it is, but within a few
years I was married and making my own life anyway. Anyway, my gratitude
to Mr. Kraft knows no bounds for providing me air cover (so to speak)
for my ground assault (I was selling these 2-lead batteries to my mates
in the club and soon enough to folks in two other clubs). This was my
first business venture (if you don't count lawn mowing and delivering
morning edition newspapers for the Birmingham News on my bike). Today?
Mr Kraft long ago flew west, his business is mothballed, and me?
Multi-lead batteries are still in our product lineup.
Now,
unlike then, two benefits accrue of having packs with multiple leads.
The first being you can use 2 on/off switches (my principal purpose way
back when). This, because it affords inexpensive redundancy. After all,
odds of both radio switches failing on the same flight are astronomical.
The
other benefit of two leads? Not a big deal back when servos only drew a
few hundred milliamps, but a really big deal, today when individual
servos easily draw several amps (1A=1000mA). Since each battery lead is
rated at 3.5A, then making two connections to the receiver through
individual leads benefits you in making 7A of current available instead
of 3.5A (before heat build up since this is a rating, not a limit). And
this is a bigger deal than most modelers realize.

Note; for propulsion, the weight and package volume
(capacity) give an overwhelming advantage to polymer bag construction.
This is why LiPo packs are used for powering RC models. But also know
this, these packs are removed prior to charging (or should be), so the
risk profile is somewhat different.

Q. At what rate should I charge my LiIon pack?
A. In general, don't exceed 5C, and best practice is charge at 1C.
Look, teaching you everything you need to know about battery charging
is beyond the scope of this website. We'll share some examples to try
and help guide you, but don't for a minute believe this is a
comprehensive explanation. Point being, it's on you to learn enough to
be safe and the charger manufacturer will also have information to use
in safely charging your battery pack.
To begin, you need to be aware of a bit of math. Like how the 'm' in mAh
means mili, or 1/1000. Regarding the A, that means Amperes (or amps),
and h=hours. So the capacity of the pack is measured amps delivered in
one hour, or Ah (A*h or A x h), but since packs for models are often
measured in fractions of an ampere, then they're also often expressed in
terms of milliamps, or 1/1000th of amp. As for time, since the time
frame is always based one hour, then . . .
- 5000mAh = 5Ah - meaning it'll deliver 5A of current for one hour
- 2000mAh = 2Ah
- 850mAh = 0.85Ah
. . . understand?
Further to this, C (capacity) of a 5Ah pack is 5A for an hour, 2.5A
for two hours. Similarly, 2A of consumption for an hour from a 2Ah pack,
get it?
So charging at 1C (meaning 1xC) it means a 5Ah pack can be charged at
5A, and if the same pack is being charged at 2C (meaning 2xC or 2x5=10)
it means it can be charged at 10A and since we're dealing with full
hours, this means it'll charge in 1/2 that much time, or 1/2 an hour.
Confused? Continue reading.
Let's switch to a 2Ah pack (same-same as a 2000mAh pack). Charging a
fully discharged 2Ah pack at 1C means hitting it with the charger at 2A
for one hour. Hit it at 2C (4A) means it takes half that much time
(1h/2=1/2h or 30-minutes) Ditto, charging at 3C takes 1/3 of an hour or
20 minutes (60min/3=20minutes), or at 4C takes 1/4th of an hour, or 15
minutes. Get it? If not, review and consider learning about this
material in other places until it clicks because if you screw up you're
putting your life at risk.
We're not kidding so please consider yourself warned!
Note; all this is in theory because it's never a good idea to
discharge below 20% capacity. Point being, a 5000mAh pack in practice
should be considered 80% of 5ooomA (0.8 x 5000=4000), or 4000mAh instead
of 5000mAh. This is important!
There's more to learn, and it's not really rocket science, but it's
on you to go learn about it before charging batteries. We're sharing
some some rules of thumb that will for the most part keep you out of
trouble - but - you can burn your house down by being stupid so don't go
trying to blame us because a) we're telling you battery charging can be
dangerous, and b) that what we're sharing isn't everything you need to
know. The major point being, you should go learn how to do it safely
before you begin!

Q. My charger has a LiPo charge-cycle instead of LiIon. May I still use it?
A. Yes.
In general, chargers expressly made to charge LiIon packs are set to
similar cell-voltages for LiPo-chemistry and thus, won't damage the
pack. Basically, the difference internally is the LiIon cell has a
liquid electrolyte while the LiPo, which is also a lithium-ion
technology, uses a gel for the electrolyte. Anyway, always use a charger
designed for the appropriate chemistry. Note; chargers are available to
charge multiple chemistries.

Q. My charger has a LiFe charge-cycle instead LiIon. May I still use it?
A. In general, no for LiIon because a LiFe-charge cycle is going to charge at a lower level than for LiIon.

Q. My battery is marked LiFePO4 instead of LiIon and marked
6.6V instead of 7.4V. My charger has a LiFe charge cycle, may I use
this?
A. Yes. Similar to how you can charge a LiIon with a LiPo
charge cycle, LiFePO4 can be charged with a LiFe charge cycle. And like
LiIon is similar to LiPo in chemistry and different in physical
construction, LiFEPO4 is similar to LiFe with similar chemistry and
different physical packaging (meaning LiFe are packaged in polymer bags
like LiPo, and LiFePO4 within metal cylindrical cells like LiIon.
And
for our purposes (handling and use within models), for pretty much the
same reason; meaning they're more robust and thus, more readily
withstand the knocks of life better. Like what? Shifting within the
fuselage during a crankshaft maneuver, maybe and if it's a soft side
construction, maybe bumping up against he hard edge of a plywood or
carbon fiber former, and getting a ding.
What's important
about this is even if you smooth the ding over with the ball of your
thumb until you can't see it, the ding damaged the electrolyte.
Forevermore, there's a damaged place (maybe now invisible to the eye)
but where heat can build up during charge and discharge.Honestly? This
could mean a fire in your workshop or home. Heads up!

Q.
I've been told LiIon are safer than LiPo and I don't have to worry
about discharging them and storing in fireproof bags. is this true?
A.
There's safer and then there's SAFER. While somewhat less prone to
spontaneously fire than LiPo battery packs, LiIon packs can and do catch
fire. What may lead to this happening? I'm not a battery designer or
chemist, in this instance this is me, John, trying to help guide you
like I was taught by those who knew more than me. So I'm sharing my
experience and trying to help you learn a few of the things I have
learned. Point being, you need to do some research on your own to
confirm pretty much anything and everything you learn, not just here,
but elsewhere. So here's what I know.
Look, chargers can
malfunction and never stop charging. This, maybe leading packs to
overheat and catch fire. We see example of LiIon fires in popular press
stories like this one in the NY Post:
Major
point being, electric scooters and bikes also use these cylindrical
cells (maybe because they fit nicely inside round steel tube frames), so
if I were you, I'd be careful about trusting *any* battery pack. Me? I
store them in fireproof bags of the type you can buy pretty much
anywhere (I buy mine off Amazon). Even then, I store these batteries
inside a .50 caliber ammo can, which I bought at an Army surplus store.
Note; I drilled a 1/4" hole in the lid (because they seal, otherwise).
This, so if one catches fire it can vent the pressure. Next, I epoxied a
bit of stainless steel wool wool on the underside (and over the hole -
but - careful not to plug it with epoxy). The purpose of this is to
serve as a smoke trap and maybe minimize the mess. Does it work? Dunno,
never had a fire. Just relating what *I* do . . . but you do you, as the
saying goes.

Q. Do I need to put my LiFePO4 battery packs at storage mode like I do my LiPo propulsion-packs and my LiIon receiver-packs?
A. No, it's our experience these are the only packs you will
own where it doesn't seem to hurt them to charge today and go fly
tomorrow, or next week, or in six-months! Like if your better-half has
other ideas and the next day while you're loading the truck with models
she announces, 'It's almost spring, take me shopping for painted Mexican terracotta pots, honey!' This, being an example of the now classic, honey-do! So you being a long suffering fellow happily married man, and well experienced in the ways of women, say, 'But of course, Dear, when do you have in mind we should go?"
Saying unlike a LiIon or LiPo which you should soon after deciding
you're not going flying connect to your charger and run a storage mode
cycle, with the LiFePO4 packs I've found you can ignore and use them
when you're good and ready.
Q. I saw a datasheet for a LiFePO4 cell and it refers to them as LiIon, what's going on with that?
A.
Technically, all cylindrical cell batteries are LiIon. It's just that
there are different types so just like there are Lithium-Iron-Phosphate
(LiFePO4 or more commonly in the industry, LFP), there are
Lithium-Manganese-Oxide (LMO), Lithium Nickle Manganese Cobalt Oxide
(NMC), Lithium-Nickle-Cobalt-Aluminum-Oxide (NCA), Lithium-Cobalt-Oxide
(LCO) and Lithium-Titanium-Oxide (LTO), and soon enough, others. And the
one thing they share is a different balance of;
- Cost
- Life Span
- Specific Energy
- Performance
- Specific Power
- Safety
The
best one for Specific Power, Safety, and Life Span is the LFP. And
because these use materials that are comparatively dirt cheap (iron and phosphate instead of nickel and cobalt),
they're less costly. And good and cheap is often a winning combination
in many things, same with batteries in my opinion. The downside is their
Performance and Specific Energy, so LFP cells have less voltage than
other types, 3.2-3.3 versus 3.7-3.8 and this makes them safer and less
prone to spontaneously catching fire.These are good things i my view,
what about yours?
But less voltage isn't as desirable as higher
voltage because servos make more torque and operate faster on higher
voltage (not just ProModeler, all servos, all brands - due to physics,
not marketing) so a caution you should bear in mind is since servos are
marketed by applying lipstick to the pig (again, all servos, not just
ours), they're rated on 8.4V instead of 6.6V because that's where they
shine!
Major point being, if you need 350oz-in and you figure
you're good because you're buying our DS360DLHV servos, think again if
you're planning on running them on a LFP pack because on 6.6V, they
won't make 350oz-in. We're very careful to disclose more than just 6.0V
and 8.4V specs and this is a perfect example of why. The DS360 is rate
at 8.4V and it gives you 360oz-in but at 6.6V it's down to 290oz-in.
Maybe good enough, maybe not. Judgement call.
But . . .

.
. . if you look instead at the specs chart for the DS415BLHV servo
(rated at 8.4V, remember), then at 6.6V it's still making 345oz-in and
that's a *lot* closer to the 350oz-in you want. But, for another ten
bucks, you can, also . . .

. . . check out the DS505BLHV servo, which at 6.6V it's still making a whopping 450oz-in. Me? I like a bit of overkill, what about you?

So the take away from this is give consideration to your servos when selecting your battery chemistry. Word to the wise.

Q. Can I charge my 2S LiIon pack with a NiCd charger for 7-cells since that's designed to charge 7.2V packs?
A. Not just no . . . but Hell no! And not just don't do it . . . don't even *think* about it.
The reason is the NiCd charger makes no provision to monitor the cell
voltage of the individual cells the way a charger designed for LiIon
packs will. This is the inherent advantage of the LiIon technology in
that each cell is wired to the little white balance-connector so the
charger can monitor the voltage of each cell as it charges. Be careful
because this is a good way to start a fire and burn down your house!
You've been warned!

Q.
I've heard if I run a LiIon or LFP pack down too low for the charger to
start the cycle, you can unplug the balance connector and set it NiCd-type in the program and it'll start right up and get some juice into the pack - enough to start the cycle with the balance connector connected.
A.
This is true. And if you do it out on the concrete of your driveway
(where if it catches fire there's less risk - but remember, you have to
explain it to the fire department and the insurance company adjuster if
things go teats up), then maybe you can get away with it. Perhaps 5-10-min, where the charger is set to charge a 7-cell NiCd pack (because 7x1.2V/cell equals about the same as an 8.4V 2S LiIon, or as a 7.2V 6-cell NiCd, again where 6x1.2V/cell mimic a 2S LFP), maybe you can get enough current into the pack to bring the voltage up enough for the automated charge cycle to take over.
So
yes, I know this may work . .. but riddle me this. What's a model
worth to you, a few hundred bucks, maybe even several thousand? And what
will you say during a deposition when opposing counsel asks, 'Sir, why would you try to save $30 resuscitating a battery, didn't you realize it could die unexpectedly, only to lose control and fly through the windshield of you buddy's parked car where his wife was crocheting booties for their granddaughter, and kill her?
Just saying, there's being smart by saving a few bucks, and then being really smart and realizing when to cut your loses. Like maybe this is one of those times when it's smarter to take the battery pack to be recycled. Just saying, I wouldn't *but* you do you because to your question, yes, folks have done this successfully.

Q. I'm Canadian and fly year around, sometimes in sub-zero temperatures. Is it OK to charge my LiIon pack in these conditions?
A. Yes, but be careful. Capacity is reduced maybe 20-30% at
freezing. And at lower temperatures the data is inconsistent. Look,
batteries basically like to 'live' at similar temperatures where 'we'
like to live. Fortunately, in practice, what most folks do is charge
their packs whilst in their car!
That said, if you do charge in below
freezing temperatures, reduce the rate of charge to 0.1C . . . e.g. 10% of
the battery capacity.

Q. I'm confused, isn't LiIon the same as LiPo? Also, why don't
you recommend LiPo packs? I like that they're cheap so what's wrong
with that?
A. Yes, LiIon and LiPo are similar. But critical differences aren't so much in their chemistry (they're actually very
similar) but in their methods of construction. This is the key to understanding our recommendation for LiIon versus LiPo.
This is because the LiPo is built in a polymer
bag. This gives it the characteristic brick shape as the
individual cells are flat-rectangles, which are overlaid upon one another. The shape is also the giveaway for
the LiPo vs. LiIon where these packs are built within cylindrical metal shells (typically aluminum).
Note; the Po in LiPo refers to the polymer in it's construction
(aluminized polymer bags). Anyway, the individual cylindrical
shells, because they're made of metal instead of thin polymer bags
means they're more resistant to physical damage. By the way, this metal
shell is the same technology used in old school NiCds and
NiMH (and alkaline cells, for that matter). It's been around forever
because it works!
There are downsides to these metal shells. First, the metal is a bit heavier than the plastic bag use in LiPos. Second, simple
geometry dictates two cylinders contain less volume than two flat cells
(capacity). Third, they're more expensive to produce.
Against these
disadvantages are upsides. Like metal shell is FAR more sturdy. This
turns out to be a crucial advantage because metal protects better
against inadvertent damage (like a pack shifting during a
maneuver and bumping up against the hard edge of a former). If this
happens to polymer style packs, the dent may result in it puffing. Or in
a fire. Need I mention our models are constructed of
flammable materials like balsa, foam, and fiberglass?
Bottom line? For an engineer, part of the remit is looking not at when
everything is
going right, but when things are going wrong. Look, nobody sets out to
install
their pack so it's dented due to shifting during a maneuver, but . . .
shit happens, right? So it's when things go pear shape that a good
engineer earns his pay.
Our deciding against continuing to offer 'Po' style packs for control
avionics is a direct result of data indicating it might sound good in
theory, but in practice, leaves something to be desired. This reminds of the immortal words of a wise wag of baseball.
Put another
way, when the data changes, we change our mind! This is why our control
avionic pack recommendation is to use durable LiIon instead of more
fragile and less costly alternatives like LiPo brick style packs.

Q. How did you come to make batteries with two leads?
A. I've mentioned how and why. before . . . now for the rest of the story.
Way
back in the day, going back on the order of 50 years ago, I flew a Lou
Andrews Aeromaster (53" wingspan). Loved that model flew it for many
years. Re-covered it twice - thank goodness for K2R spray. Powered by my
Lee Custom K&B .61 on SIG 5% with 2% added castor and turning a
12x6, it was a delightful model until it met an untimely demise due to
switch failure. This crash led me to develop a two-lead battery pack.
This
all aided by Mr. Kraft of Kraft Systems who generously agreed to sell
me 25 of their 4-cell NiCd 500mAh battery packs, to include 25 extra
leads (no other source for a Multicon connector since that was their
proprietary product), and he even included a sheet of gold foil labels
with which to reseal the case halves like brand new for after I soldered
on the 2nd lead. I was on my own for finding a larger rubber grommet
but he even lent me a hand with this by including a small bag of servo
mounting grommets. And he gave me some round Kraft patches to hand out,
too.
All this in part because (and I am reading into his actions
what I *think* were the reasons). Meaning, I think he, a) didn't want to
complicate his life with another SKU (stock keeping unit in the
parlance of product sales), and b) believed his switches were reliable
enough, and looking back, c) it maybe tickled him to help start someone
(me) get a start in a business career.
Me? I will remain forever
grateful to his largess to a snot nose kid with a business idea because
he could have blown me off. Unfortunately, when I went off to college,
my mom wanted the room for whatever and tossed pretty everything that
remained of my life there. By this meaning my paperback science fiction
books (I've subsequently replaced my Heinlein, Clark,and Asimov and
added to them the rest in print (except Asimov as I don't have that much
money). She also tossed things like my sandpaper collection stored
within an accordion file my grandfather had given me (embossed with his
law office logo, and of course, irreplaceable), plus my correspondence
with Mr. Kraft, which at the time i didn't attach much importance to. So
basically, pretty much everything I hadn't taken with me to university -
to include my modeling tools - like sanding blocks, X-Acto, Zona saw,
model razor plane, workbench, etc. went into the ash bin as she
basically erased me like I was never there. And looking back, this
sounds bad but she wanted the room and all my stuff looked like just
trash to her broom!
Sounds worse than it is, but within a few
years I was married and making my own life anyway. Anyway, my gratitude
to Mr. Kraft knows no bounds for providing me air cover (so to speak)
for my ground assault (I was selling these 2-lead batteries to my mates
in the club and soon enough to folks in two other clubs). This was my
first business venture (if you don't count lawn mowing and delivering
morning edition newspapers for the Birmingham News on my bike). Today?
Mr Kraft long ago flew west, his business is mothballed, and me?
Multi-lead batteries are still in our product lineup.
Now,
unlike then, two benefits accrue of having packs with multiple leads.
The first being you can use 2 on/off switches (my principal purpose way
back when). This, because it affords inexpensive redundancy. After all,
odds of both radio switches failing on the same flight are astronomical.
The
other benefit of two leads? Not a big deal back when servos only drew a
few hundred milliamps, but a really big deal, today when individual
servos easily draw several amps (1A=1000mA). Since each battery lead is
rated at 3.5A, then making two connections to the receiver through
individual leads benefits you in making 7A of current available instead
of 3.5A (before heat build up since this is a rating, not a limit). And
this is a bigger deal than most modelers realize.

Note; for propulsion, the weight and package volume
(capacity) give an overwhelming advantage to polymer bag construction.
This is why LiPo packs are used for powering RC models. But also know
this, these packs are removed prior to charging (or should be), so the
risk profile is somewhat different.
